Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ibe, Junya*; Aso, Megumi*; Takahatake, Yoko; Watanabe, So; Watanabe, Masayuki; Matsuura, Haruaki*
no journal, ,
Since waste salt generated from pyro-reprocessing test which contains uranium can easily capture moisture and corrode equipment, further treatment technology for decontamination. Oxides are added as oxygen donor in the melts, and then uranium is separated from the salt as precipitates. In the next step, melt bath components are evaporated by a vacuum distillation. First, LiCl-KCl eutectic and NaCl-2CsCl salts were used as melt baths, lithium oxide was used as a precipitant, and cerium chloride was used as uranium surrogate for testing the precipitation process. Next, a distillation line has been constructed and the best condition for distillation has been searched. Amount of the precipitates increased with increasing the amount of oxide, and recovery ratio of cerium must potentially depend on solubility of oxychloride into the bath salts. It is considered that oxychloride was formed by the similarity in EXAFS oscillation and X-ray diffraction patterns.
Hayashi, Hirokazu; Minato, Kazuo*
no journal, ,
For a basis of the future nuclear fuel cycle, understanding the behavior of transuranium elements (TRU: Np, Pu, Am, Cm) is important. Experimental study on pyrochemical reprocessing of the fuels containing TRU is one of the important topics. But limited data are available on the behavior of TRU in some molten salts including NaCl-2CsCl melt which is considered to be used for pyrochemical reprocessing of MOX fuels in RIAR process. Only the electrochemical behavior of Np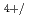 Np
Np redox couple has been reported for Np ions in NaCl-2CsCl melt. In the present study, behavior of Np ions including the reduction of Np
redox couple has been reported for Np ions in NaCl-2CsCl melt. In the present study, behavior of Np ions including the reduction of Np in NaCl-2CsCl melt was investigated. A typical cyclic voltammogram shows 2 groups of the signals corresponding to Np species. They were assigned to the redox reactions of Np
in NaCl-2CsCl melt was investigated. A typical cyclic voltammogram shows 2 groups of the signals corresponding to Np species. They were assigned to the redox reactions of Np /Np and Np
/Np and Np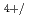 Np
Np , respectively by comparing with those observed in LiCl-KCl melt. The redox potentials and the diffusion coefficients obtained by various transient electrochemical techniques will be presented.
, respectively by comparing with those observed in LiCl-KCl melt. The redox potentials and the diffusion coefficients obtained by various transient electrochemical techniques will be presented.